Bohr rutherford diagram first 20 elements – The Bohr-Rutherford diagram, a pioneering model of atomic structure, stands as a testament to the scientific ingenuity that has shaped our understanding of the fundamental building blocks of matter. This groundbreaking diagram, conceived by Niels Bohr and Ernest Rutherford, provides a conceptual framework for visualizing the arrangement of electrons within the first 20 elements, laying the groundwork for modern chemistry and physics.
The Bohr-Rutherford diagram not only revolutionized our comprehension of atomic structure but also paved the way for advancements in various scientific disciplines. Its enduring legacy lies in its ability to elucidate complex atomic phenomena, from chemical bonding to the intricacies of atomic spectroscopy.
As we delve into the intricacies of this diagram, we will explore its historical significance, key components, and far-reaching applications.
Bohr-Rutherford Diagram: A Conceptual Overview
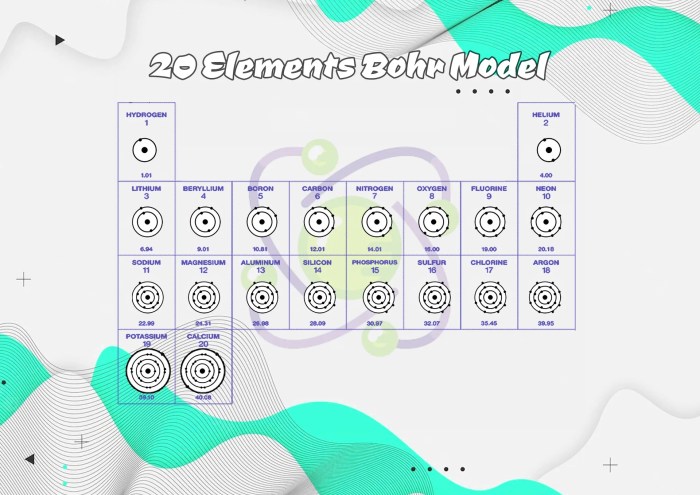
The Bohr-Rutherford diagram, developed in the early 20th century, revolutionized our understanding of atomic structure. This conceptual model depicted the atom as a central nucleus surrounded by orbiting electrons, providing a foundation for subsequent advancements in atomic theory.
Key components of the diagram include the nucleus, containing protons and neutrons, and electrons arranged in concentric energy levels around the nucleus. The energy levels, labeled K, L, M, and so on, represent the quantized energy states that electrons can occupy.
While the Bohr-Rutherford diagram provided a groundbreaking framework, it had limitations. It could not fully explain the behavior of electrons in more complex atoms or account for certain atomic phenomena. However, it paved the way for the development of more sophisticated atomic models, including the quantum mechanical model.
Electron Arrangement and Energy Levels
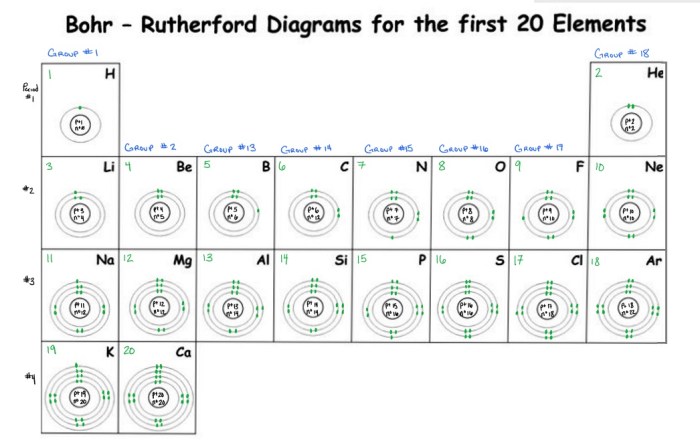
The Bohr-Rutherford diagram provides a systematic arrangement of electrons within the first 20 elements. Electrons fill energy levels in order of increasing energy, starting with the lowest energy level, K.
- The number of electrons in each energy level is determined by the quantum numbers, which describe the electron’s energy, angular momentum, and spin.
- Electron configuration, the distribution of electrons across energy levels, influences an element’s chemical properties.
- For example, elements with a filled outermost energy level, such as helium, are chemically inert, while elements with partially filled outermost energy levels, such as sodium, are highly reactive.
Applications in Chemistry and Physics: Bohr Rutherford Diagram First 20 Elements
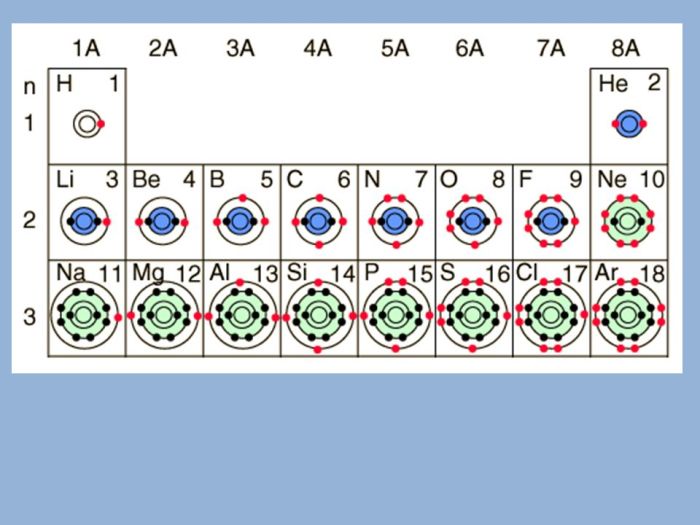
The Bohr-Rutherford diagram has wide-ranging applications in chemistry and physics:
- Atomic Spectroscopy:The diagram explains the emission and absorption of light by atoms, providing insights into atomic structure and energy transitions.
- Chemical Bonding:It helps visualize the electron sharing and transfer involved in chemical bonding, explaining the formation of molecules and compounds.
- Material Properties:The arrangement of electrons in energy levels influences the electrical, optical, and magnetic properties of materials.
Comparison with Modern Atomic Models
The Bohr-Rutherford diagram is a stepping stone in the evolution of atomic theory. Compared to modern quantum mechanical models, it has limitations:
- Oversimplified Electron Orbits:The diagram depicts electrons as orbiting the nucleus in fixed circular paths, while quantum mechanics reveals that electron behavior is probabilistic and described by wave functions.
- Inadequacy for Complex Atoms:It cannot fully explain the behavior of electrons in larger, more complex atoms.
However, the Bohr-Rutherford diagram remains valuable as a conceptual tool for understanding the basic principles of atomic structure.
Educational Value and Impact
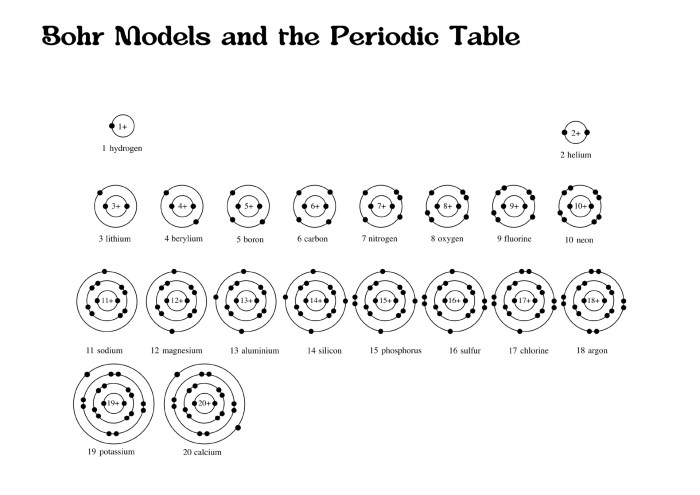
The Bohr-Rutherford diagram is an indispensable educational tool:
- Visual Representation:It provides a tangible visualization of atomic structure, making it easier for students to understand complex concepts.
- Foundation for Advanced Concepts:It serves as a stepping stone to introduce more sophisticated atomic models, such as the quantum mechanical model.
- Historical Significance:The diagram highlights the evolution of scientific thought and the contributions of Bohr and Rutherford to our understanding of the atom.
FAQ Insights
What is the significance of the Bohr-Rutherford diagram?
The Bohr-Rutherford diagram marked a significant advancement in our understanding of atomic structure, providing a conceptual framework for visualizing the arrangement of electrons within the first 20 elements. It laid the foundation for modern chemistry and physics, enabling scientists to explain various atomic phenomena.
How does the Bohr-Rutherford diagram explain electron arrangement?
The Bohr-Rutherford diagram depicts electrons orbiting the nucleus in concentric circular paths, known as energy levels. Each energy level can accommodate a specific number of electrons, and the arrangement of electrons within these levels determines the chemical properties of the element.
What are the limitations of the Bohr-Rutherford diagram?
While the Bohr-Rutherford diagram provides a simplified model of atomic structure, it has certain limitations. It does not account for the wave-particle duality of electrons, the existence of subshells within energy levels, or the complex interactions between electrons.