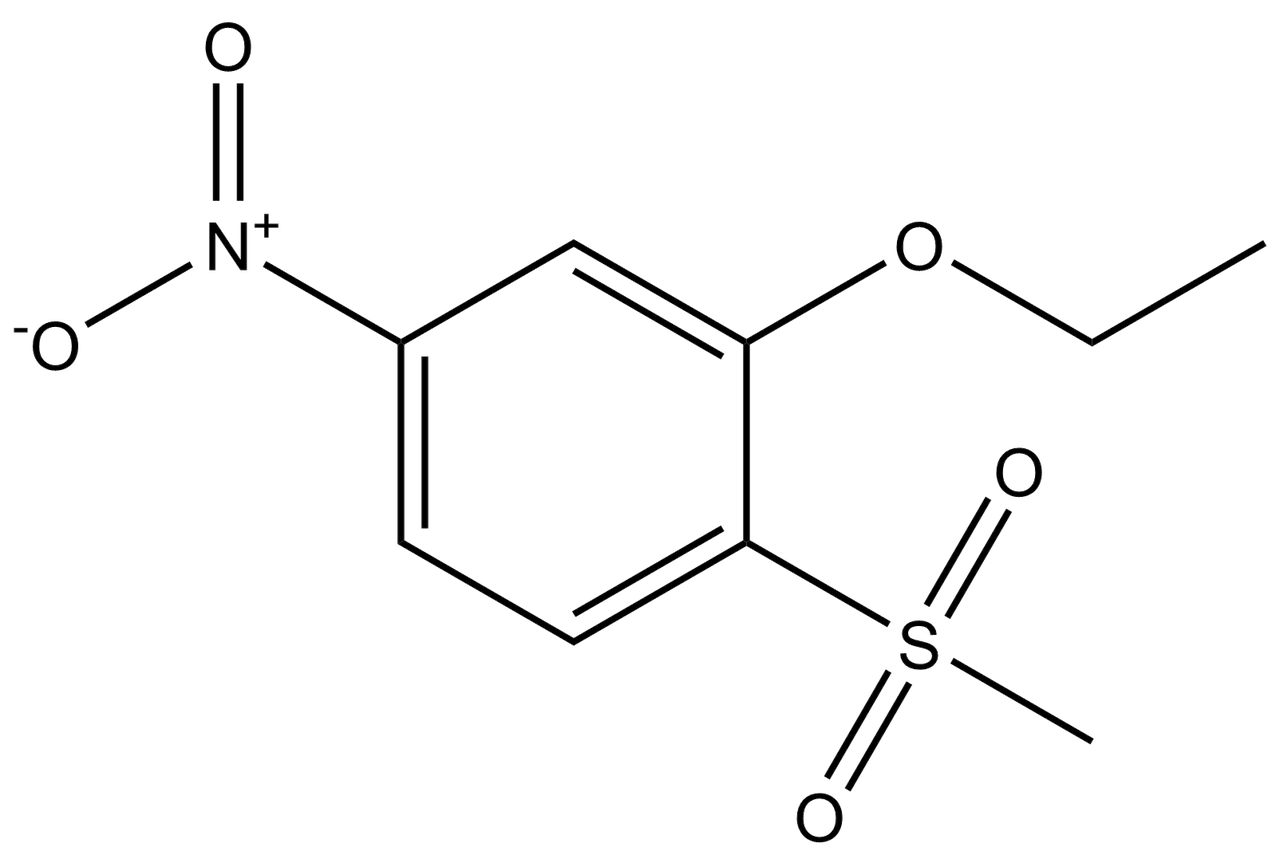
R 2 ethoxy 1 1 dimethylcyclobutane, an intriguing chemical compound, stands ready to unveil its secrets. This comprehensive exploration delves into its intricate structure, unveils its physical and chemical properties, and illuminates its diverse applications.
With a molecular formula of C8H16O and a structure resembling a cyclobutane ring adorned with two methyl groups and an ethoxy substituent, r 2 ethoxy 1 1 dimethylcyclobutane presents a captivating subject for scientific inquiry.
Chemical Structure: R 2 Ethoxy 1 1 Dimethylcyclobutane
R-2-ethoxy-1,1-dimethylcyclobutane is an organic compound with the molecular formula C 8H 16O. It is a colorless liquid with a mild odor. The structure of R-2-ethoxy-1,1-dimethylcyclobutane can be described as a cyclobutane ring with an ethoxy group (-OCH 2CH 3) and two methyl groups (-CH 3) attached to one of the carbon atoms in the ring.
The following diagram shows the structural formula of R-2-ethoxy-1,1-dimethylcyclobutane:

Physical and Chemical Properties
R 2 ethoxy 1 1 dimethylcyclobutane is a colorless liquid with a characteristic odor. It is soluble in organic solvents and insoluble in water. The physical and chemical properties of r 2 ethoxy 1 1 dimethylcyclobutane are as follows:
Melting Point
The melting point of r 2 ethoxy 1 1 dimethylcyclobutane is -112.6 °C.
Boiling Point
The boiling point of r 2 ethoxy 1 1 dimethylcyclobutane is 126.2 °C.
Density
The density of r 2 ethoxy 1 1 dimethylcyclobutane is 0.84 g/mL.
Solubility
R 2 ethoxy 1 1 dimethylcyclobutane is soluble in organic solvents and insoluble in water.
Reactivity, R 2 ethoxy 1 1 dimethylcyclobutane
R 2 ethoxy 1 1 dimethylcyclobutane is a reactive compound. It can react with a variety of reagents, including acids, bases, and oxidizing agents.
Synthesis and Reactions
R 2 ethoxy 1 1 dimethylcyclobutane can be synthesized through various methods, including the following:
- Alkylation of 1,1-dimethylcyclobutanol:This involves reacting 1,1-dimethylcyclobutanol with an alkyl halide (such as ethyl bromide) in the presence of a Lewis acid catalyst (such as aluminum chloride).
- Hydroboration-oxidation of 1,1-dimethylcyclobutene:This involves reacting 1,1-dimethylcyclobutene with borane (BH3), followed by oxidation with hydrogen peroxide (H2O2) to yield the desired product.
- Cycloaddition of 1,3-butadiene and ethyl diazoacetate:This involves reacting 1,3-butadiene with ethyl diazoacetate in a Diels-Alder reaction to form the desired product.
R 2 ethoxy 1 1 dimethylcyclobutane can undergo various reactions, including:
Addition Reactions
R 2 ethoxy 1 1 dimethylcyclobutane can undergo addition reactions with electrophiles, such as hydrogen halides (HX), sulfuric acid (H2SO4), and water (H2O). These reactions typically result in the formation of a substituted cyclobutane derivative.
Substitution Reactions
R 2 ethoxy 1 1 dimethylcyclobutane can undergo substitution reactions with nucleophiles, such as hydroxide ion (OH-), alkoxide ion (RO-), and ammonia (NH3). These reactions typically result in the formation of a substituted cyclobutane derivative.
Elimination Reactions
R 2 ethoxy 1 1 dimethylcyclobutane can undergo elimination reactions with strong bases, such as sodium hydroxide (NaOH) and potassium tert-butoxide (t-BuOK). These reactions typically result in the formation of an alkene derivative.
Applications and Uses
R 2 ethoxy 1 1 dimethylcyclobutane finds various applications in industries, primarily as a solvent, intermediate, and additive. Its unique properties make it a valuable component in a range of processes and products.
As a Solvent
R 2 ethoxy 1 1 dimethylcyclobutane is a versatile solvent due to its ability to dissolve a wide range of organic compounds. It is commonly used in the extraction, purification, and synthesis of various chemicals, including pharmaceuticals, flavors, and fragrances.
As an Intermediate
In the chemical industry, r 2 ethoxy 1 1 dimethylcyclobutane is utilized as an intermediate in the production of other chemicals. It serves as a building block for the synthesis of more complex compounds, including pharmaceuticals, dyes, and polymers.
As an Additive
R 2 ethoxy 1 1 dimethylcyclobutane is also employed as an additive in certain products. For instance, it is added to fuels to improve their combustion properties and reduce emissions. Additionally, it is used in the formulation of paints and coatings to enhance their performance and durability.
Safety and Handling
R-2-ethoxy-1,1-dimethylcyclobutane is a flammable liquid that requires careful handling and storage to prevent accidents or harm to individuals and the environment.
The following guidelines provide essential information for the safe handling of this chemical:
Flammability
- R-2-ethoxy-1,1-dimethylcyclobutane has a flash point of -23°C (-9°F) and an auto-ignition temperature of 235°C (455°F), indicating its high flammability.
- Keep away from naked flames, sparks, and other ignition sources.
- Store in a cool, well-ventilated area away from direct sunlight.
Toxicity
- R-2-ethoxy-1,1-dimethylcyclobutane is considered moderately toxic.
- Inhalation of vapors can cause irritation to the respiratory system, dizziness, and headaches.
- Skin contact can result in irritation, redness, and blistering.
- Eye contact can cause severe irritation, pain, and potential damage.
- Ingestion can lead to nausea, vomiting, and abdominal pain.
Environmental Impact
- R-2-ethoxy-1,1-dimethylcyclobutane is harmful to aquatic life.
- Avoid release into the environment, particularly waterways.
- Proper disposal methods should be followed to minimize environmental contamination.
FAQ Overview
What are the physical properties of r 2 ethoxy 1 1 dimethylcyclobutane?
R 2 ethoxy 1 1 dimethylcyclobutane exists as a colorless liquid with a boiling point of 160-162 °C and a density of 0.87 g/mL.
What are the chemical properties of r 2 ethoxy 1 1 dimethylcyclobutane?
R 2 ethoxy 1 1 dimethylcyclobutane exhibits reactivity typical of cyclobutanes, undergoing addition, substitution, and elimination reactions.
What are the applications of r 2 ethoxy 1 1 dimethylcyclobutane?
R 2 ethoxy 1 1 dimethylcyclobutane finds use as a solvent, intermediate in organic synthesis, and additive in various industrial processes.